|
||||||
 |
||||||
|
|
|||||
|
|
|
OM-BIOCHIP (PSA)
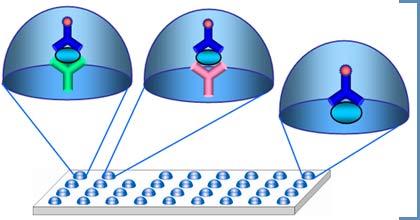
Biochip-based diagnostic kit «OM-Biochip (PSA)» for quantitative determination of PSA, total and free forms, in blood serum by immunofluorescent method. Advantages of «OM-BIOCHIP(PSA)» test-systemBiochip-based assay of two forms of PSA is carried out in «one patient - one biochip» format. Sensitivity of detection is 0.3 ng/ml for PSA, total form, and 0.3 ng/ml for PSA, free form. Variation coefficient (CV) for the measurements within one batch of biochips is not higher than 10%. Operation principle - sandwich immunoassay with fluorescent detection Biochip contains gel elements with immobilized monoclonal antibodies against PSAtotal and PSAfree (4 identical elements in a row). Immobilized antibodies against PSAtotal recognize both PSA in a free form and PSA in a complex with α1-antichymotrypsin. Immobilized antibodies against PSAfree bind PSA in a free form only. Fluorescently labeled monoclonal antibodies which are able to bind both free PSA and PSA in a complex with anti-chymotrypsin are used as developing antibodies. The intensity of fluorescence signals from biochip gel elements containing antibodies is proportional to the concentration of PSA in a sample.
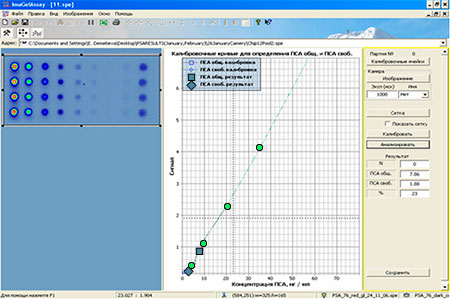
Assay protocol for «OM-BIOCHIP (PSA)» test-system
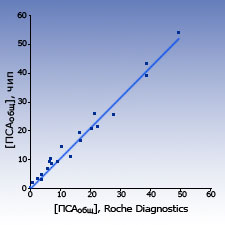
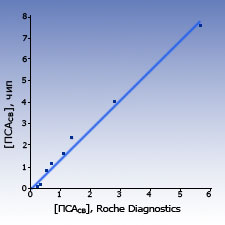
Comparison with other test-systems The results of measurements of PSAtotal and PSAfree concentration in blood sera samples of healthy donors and patients obtained with biochips were close to the results obtained by the standard ELISA systems.
Composition of «OM-BIOCHIP(PSA)» test-system
|
|
Why it is important? Prostate-specific antigen (PSA) — serological marker of prostate cancer, one of the most widespread type of cancer in men. PSA is a highly specific tumor marker. Determination of concentration of two forms of PSA and their ratio in a blood serum of a patient allows carrying out differential diagnosing of malignant and benign tumors of prostate.
Prostate cancer morbidity in Russia
(Cases per 100,000 people)
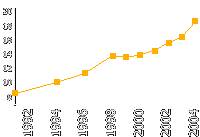
Group of risk — men aged 50 and above.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||